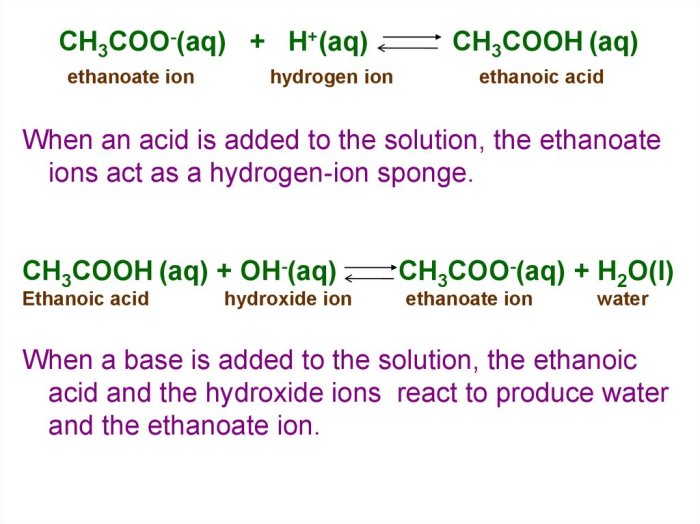
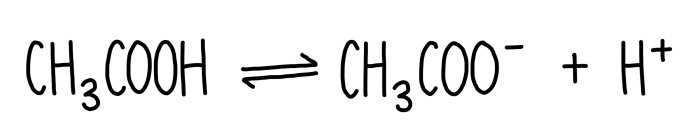The dissociation of ethanoic acid is represented above – The dissociation of ethanoic acid is a fundamental chemical process that plays a crucial role in various scientific and industrial applications. This comprehensive guide delves into the intricacies of ethanoic acid dissociation, exploring its mechanism, equilibrium constant, applications, and safety considerations.
Ethanoic acid, also known as acetic acid, is a weak acid that undergoes dissociation in aqueous solutions. The dissociation process involves the breaking of the covalent bond between the hydrogen and oxygen atoms, resulting in the formation of hydrogen ions (H+) and acetate ions (CH3COO-).
The extent of dissociation is influenced by several factors, including temperature, concentration, and the presence of other ions.
The Dissociation of Ethanoic Acid: The Dissociation Of Ethanoic Acid Is Represented Above
The dissociation of ethanoic acid is a chemical reaction in which ethanoic acid (CH 3COOH) breaks down into hydrogen ions (H +) and acetate ions (CH 3COO –).
Chemical Equation
The chemical equation for the dissociation of ethanoic acid is:
CH 3COOH(aq) ⇌ H +(aq) + CH 3COO –(aq)
Factors Affecting Dissociation
The dissociation of ethanoic acid is affected by several factors, including:
- Concentration of ethanoic acid
- Temperature
- Solvent
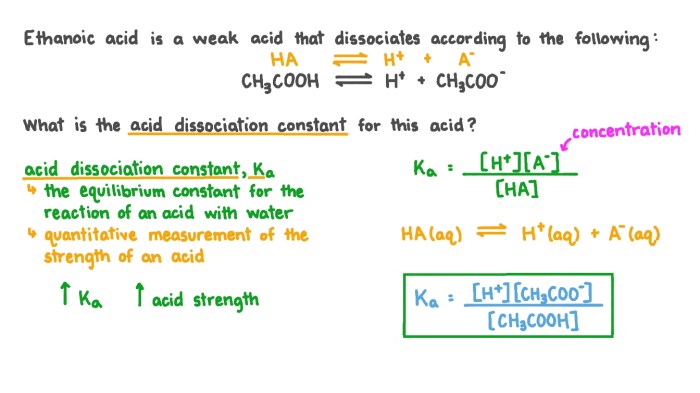
The Equilibrium Constant for the Dissociation of Ethanoic Acid, The dissociation of ethanoic acid is represented above
The equilibrium constant (K a) for the dissociation of ethanoic acid is a measure of the extent to which the acid dissociates.
Calculation of Equilibrium Constant
The equilibrium constant can be calculated using the following equation:
K a= [H +][CH 3COO –]/[CH 3COOH]
Factors Affecting Equilibrium Constant
The equilibrium constant for the dissociation of ethanoic acid is affected by several factors, including:
- Temperature
- Solvent
- Ionic strength
Applications of the Dissociation of Ethanoic Acid
The dissociation of ethanoic acid has numerous applications in everyday life and industry.
Everyday Applications
- Vinegar
- Food preservation
- Cleaning
Industrial Applications
- Production of acetic anhydride
- Production of vinyl acetate
- Textile industry
Safety Considerations for the Dissociation of Ethanoic Acid
Ethanoic acid is a corrosive substance that can cause skin burns and eye damage.
Hazards
- Corrosive
- Irritant
- Flammable
Safety Precautions
- Wear protective clothing
- Handle in a well-ventilated area
- Avoid contact with skin and eyes
Disposal
Ethanoic acid should be disposed of according to local regulations.
FAQ Section
What is the chemical equation for the dissociation of ethanoic acid?
CH3COOH(aq) + H2O(l) <=> H3O+(aq) + CH3COO-(aq)
What factors affect the dissociation of ethanoic acid?
Temperature, concentration, and the presence of other ions.
What are some applications of the dissociation of ethanoic acid?
Vinegar production, food preservation, and textile manufacturing.
What safety precautions should be taken when working with ethanoic acid?
Wear gloves, eye protection, and a lab coat. Avoid contact with skin and eyes.