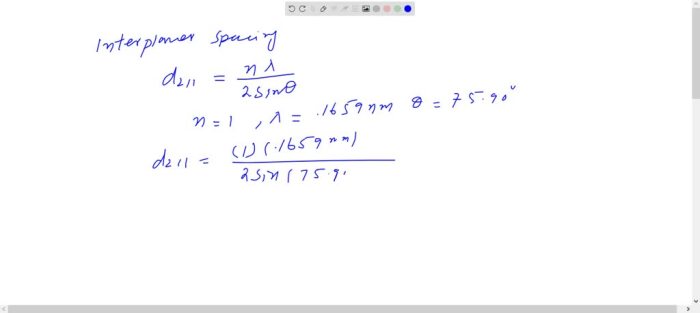
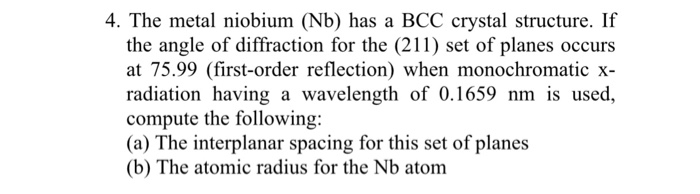
Niobium has a bcc crystal structure – Niobium, a metal with a body-centered cubic (BCC) crystal structure, exhibits unique properties and applications due to its atomic arrangement. Understanding the BCC structure of niobium is crucial for comprehending its physical and chemical characteristics.
The BCC structure consists of atoms arranged at the corners and the center of a cube, resulting in a high packing density. This arrangement influences niobium’s properties, such as its high strength, ductility, and electrical conductivity.
Niobium’s Crystal Structure

Niobium exhibits a body-centered cubic (BCC) crystal structure, a common arrangement in metals. In a BCC structure, atoms are arranged in a cubic lattice with one atom at each corner and one atom in the center of the cube.
Atomic Arrangement in a BCC Structure
The BCC structure consists of a unit cell with eight atoms located at the corners and one atom in the center. Each corner atom is shared by eight adjacent unit cells, while the central atom is shared by six adjacent unit cells.
Properties of Niobium, Niobium has a bcc crystal structure
The BCC structure of niobium influences its physical and chemical properties.
- Density:Niobium has a density of 8.57 g/cm³, which is relatively high compared to other metals.
- Melting Point:The melting point of niobium is 2,477 °C (4,491 °F), indicating its high thermal stability.
- Electrical Conductivity:Niobium has a low electrical conductivity compared to other metals, due to the scattering of electrons by the BCC structure.
Applications of Niobium
Niobium’s BCC structure makes it suitable for various applications.
- Superconductivity:Niobium is used in superconducting magnets due to its high critical magnetic field and low electrical resistance.
- Aerospace:Niobium is utilized in aerospace applications, such as jet engines and rocket nozzles, due to its high-temperature strength and oxidation resistance.
- Medical Devices:Niobium is used in medical implants and surgical instruments because of its biocompatibility and corrosion resistance.
Comparison with Other Metals
The BCC structure of niobium differs from other common crystal structures found in metals.
- Face-Centered Cubic (FCC):FCC metals, such as copper and aluminum, have atoms arranged in a cubic lattice with atoms at each corner and in the center of each face.
- Hexagonal Close-Packed (HCP):HCP metals, such as titanium and zinc, have atoms arranged in a hexagonal lattice with layers of atoms stacked in an alternating pattern.
Advanced Applications
The unique properties of niobium’s BCC structure make it suitable for advanced applications.
- Energy Storage:Niobium is being explored for use in high-capacity energy storage devices due to its ability to form stable intermetallic compounds.
- Aerospace:Niobium alloys are used in high-performance aerospace components, such as turbine blades and rocket engines, due to their exceptional strength and heat resistance.
- Medical Devices:Niobium-based biomaterials are being developed for orthopedic implants and surgical instruments, offering improved biocompatibility and mechanical properties.
FAQ Corner: Niobium Has A Bcc Crystal Structure
What is the significance of niobium’s BCC crystal structure?
The BCC structure contributes to niobium’s high strength, ductility, and electrical conductivity, making it suitable for various applications.
How does the BCC structure influence niobium’s properties?
The BCC structure results in a high packing density, which affects niobium’s mechanical and electrical properties.
In which industries is niobium commonly used due to its BCC structure?
Niobium is utilized in aerospace, electronics, and energy storage industries due to its unique properties arising from its BCC structure.